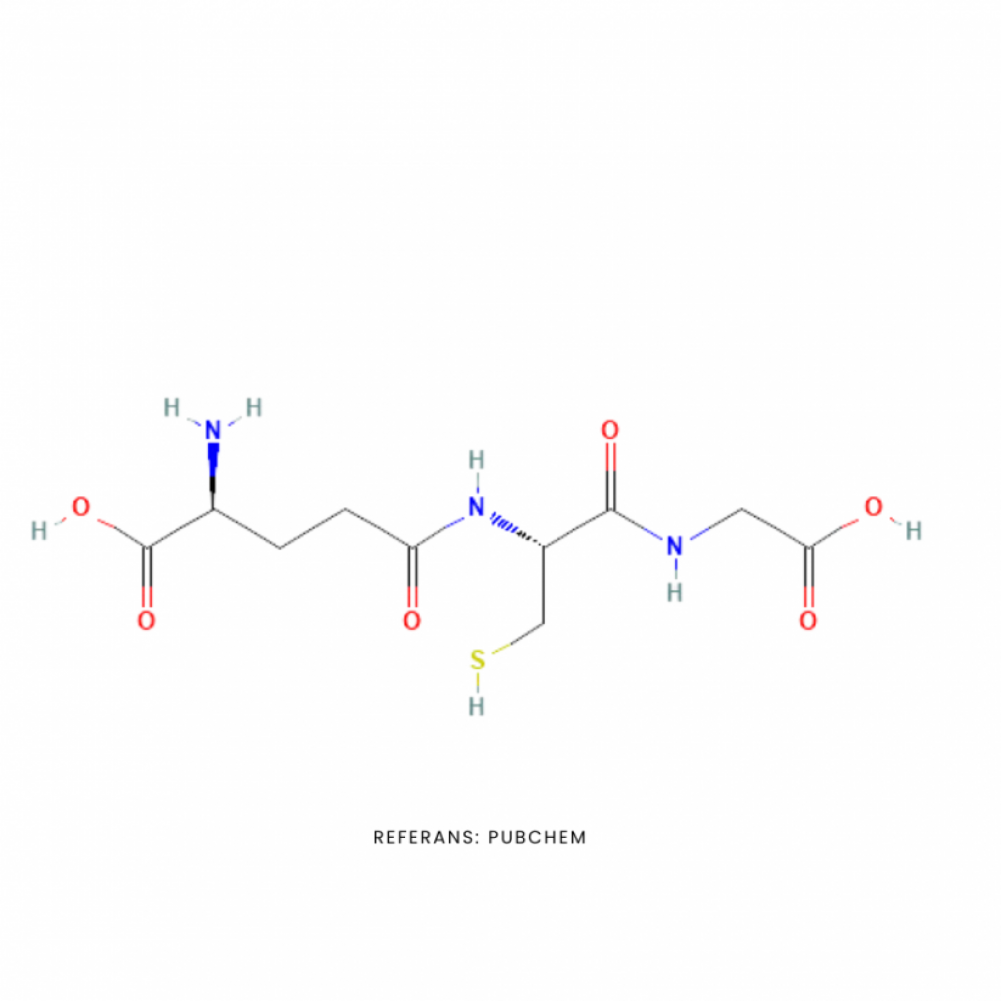
Glutathione is a tripeptide consisting of three amino acids: cysteine, glycine and glutamate, which exists in the cell in two forms, reduced and oxidized, and is a very powerful antioxidant. Glutathione is an antioxidant that directly scavenges various oxidants such as superoxide anion, hydroxyl radical, nitric oxide and carbon radicals. It is a cofactor for many antioxidants. It plays a role in the regeneration of vitamin C and vitamin E. It provides neutralization of free radicals produced by chemical toxins belonging to liver metabolism. It is involved in the transport of mercury from cells and brain. It is very important for the regulation of cellular proliferation and apoptosis, as well as for mitochondrial function and the formation of mitochondrial DNA.
Sequence: XCG
Molecular Formula: C10H17N3O6S
Biological Description: H-ꝩGlu-Cys-Gly-OH
Molecular Weight: 307.33 g/mol
PubChem CID: 124886
CAS Number: 70-18-8
Synonyms: L-Glutathione reduced, reduced glutathione
MeSH Pharmacological Classification: A tripeptide with many roles in cells. It conjugates to drugs to make them more soluble for excretion, is a cofactor for some enzymes, is involved in protein disulfide bond rearrangement and reduces peroxides.
ATC Code:
V Drugs Used for Different Purposes
V03 All Other Therapeutic Products
V03A All Other Therapeutic Products
V03AB Antidotes
V03AB32 Glutathione
Glutathione (GSH) participates in leukotriene synthesis and is a cofactor for the enzyme glutathione peroxidase. It also plays a role in the hepatic biotransformation and detoxification process; it acts as a hydrophilic molecule that is added to other lipophilic toxins or wastes prior to entering biliary excretion. It participates in the detoxification of methylglyoxal, a toxic by-product of metabolism, mediated by glyoxalase enzymes. Glyoxalase I catalyzes the conversion of methylglyoxal and reduced glutathione to S-D-Lactoyl-glutathione. Glyoxalase II catalyzes the conversion of S-D-Lactoyl Glutathione to Reduced Glutathione and D-lactate. Glyoxalase I catalyzes the conversion of methylglyoxal and reduced glutathione to S-D-Lactoyl-glutathione. Glyoxalase II catalyzes the conversion of S-D-Lactoyl Glutathione to Reduced Glutathione and D-lactate. GSH is a cofactor of conjugation and reduction reactions that are catalyzed by glutathione S-transferase enzymes expressed in the cytosol, microsomes, and mitochondria. However, it is capable of participating in non-enzymatic conjugation with some chemicals, as it is hypothesized to do to a significant extent with n-acetyl-p-benzoquinone imine (NAPQI), the reactive cytochrome P450 reactive metabolite formed by toxic overdose of acetaminophen. Glutathione in this capacity binds to NAPQI as a suicide substrate and in the process detoxifies it, taking the place of cellular protein sulfhydryl groups which would otherwise be toxically adducted. The preferred medical treatment to an overdose of this nature, whose efficacy has been consistently supported in literature, is the administration (usually in atomized form) of N-acetylcysteine, which is used by cells to replace spent GSSG and allow a usable GSH pool.